Dr. Jia-Wei Hsu

- Journal Papers
- Degrees and Positions Held
- Research
| School Name | Department | Degree | Period |
| Academia Sinica | Institute of Biological Chemistry | Joint Appointment Assistant Research Fellow | 2020-present |
| National Taiwan University | Institute of Biochemical Sciences | Assistant Professor | 2020-present |
| National Taiwan University | Institute of Molecular Medicine | Visiting Specialist | 2019-2020 |
| Harvard Medical School and Brigham & Women’s Hospital | Postdoctoral Research Fellow | 2015-2019 | |
| National Taiwan University | Institute of Molecular Medicine | Postdoctoral Research Fellow | 2013-2015 |
| National Taiwan University | Institute of Molecular Medicine | Ph.D. | 2008-2013 |
| Youtube► | Personal Video |
| Youtube► | Summer Internship |
Molecular Mechanism of Protein Transport
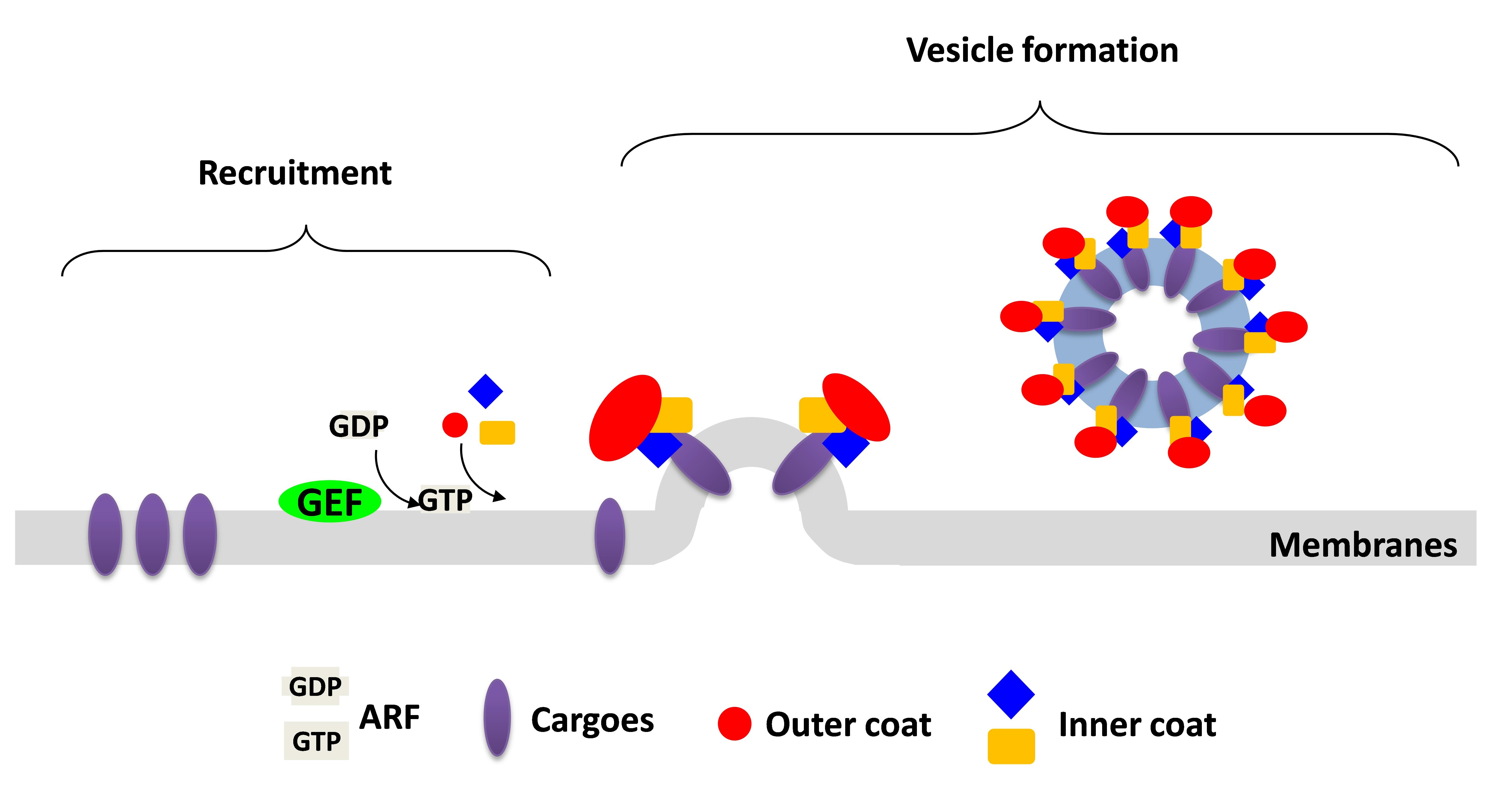
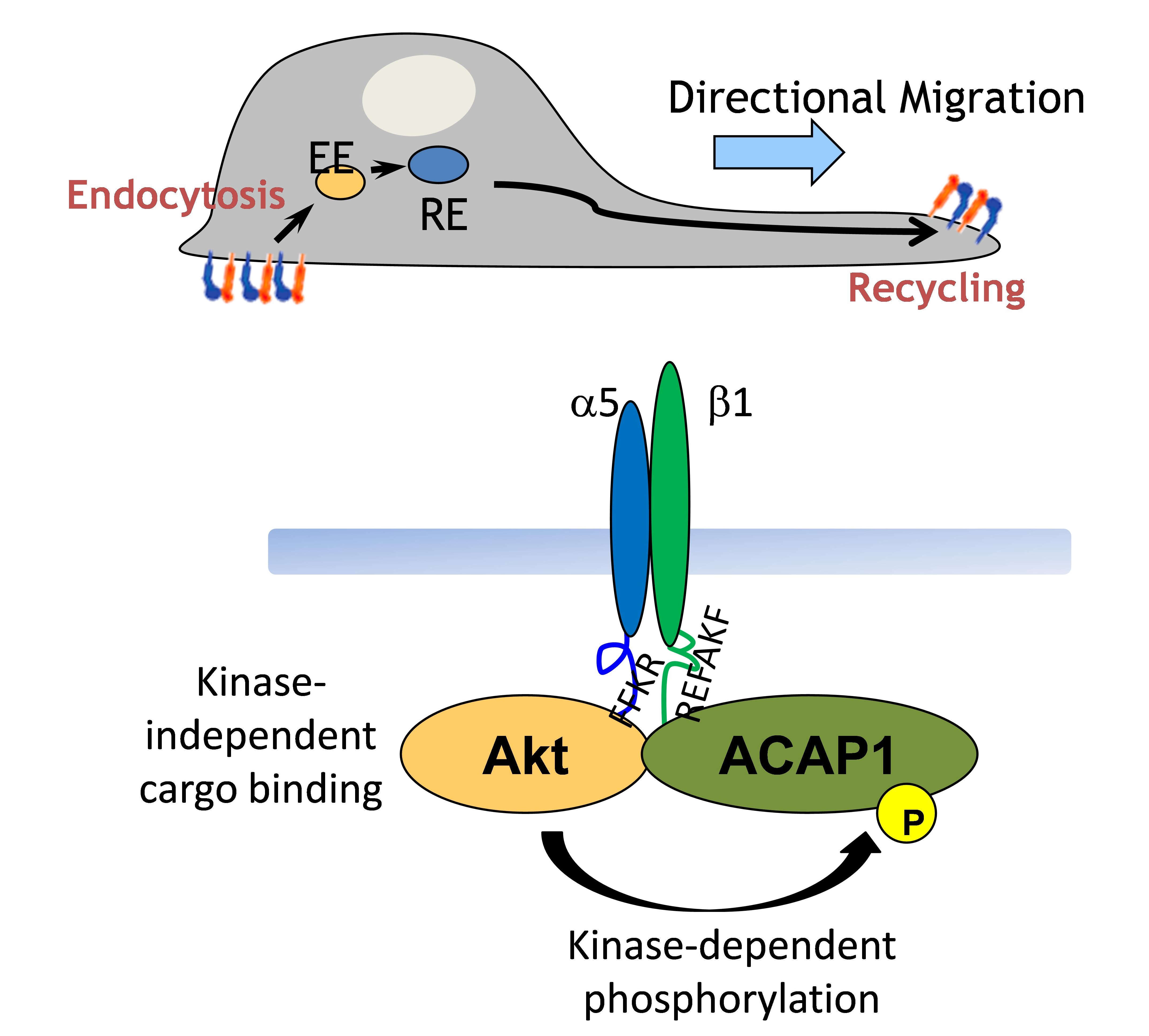
My research focuses on how intracellular protein transport is tightly regulated. Coat proteins, which initiate intracellular transport by coupling their roles in the vesicle formation and cargo sorting, are the best described to control the vesicular transport through their abilities to deform the membrane and directly bind to the cargo. ARF6 (ADP-Ribosylation Factor 6) is a small GTPase functioning in the regulation of endocytic recycling. Previously, I already defined the cargo adaptors, ACAP1 and AKT, both required for the endocytic recycling. ACAP1 (ARFGAP with Coil-coil, Ankyrin repeat and PH domain 1) is a GAP (GTPase-Activating Protein) for ARF6 to promote GTP hydrolysis of ARF6 and therefore to release ARF6 from the membrane. AKT is a serine/threonine kinase involving in many cellular processes, such as cell growth and migration. Although ACAP1 and AKT work together to regulate endocytic recycling, however, the upstream signaling that facilitates ACAP1 and AKT for the function of endocytic recycling remains unclear. In addition, how ACAP1 and AKT cooperate each other to form the complex for cargo sorting still needs to be resolved. These functional studies on the coat protein complexes will shed molecular insights into key physiologic and pathologic events, such as cell growth and migration.
Cargo Sorting during Vesicle Formation
Coat proteins play a major role in vesicular transport by binding to cargoes and sorting into intracellular compartment. Cargo recognition is mediated by components of the coat complex known as adaptor proteins. We previously showed that Arf-GAP with coil-coil, ANK repeat and PH domain-containing protein 1 (ACAP1) functions as an adaptor for a clathrin coat complex to regulate endocytic recycling. We further demonstrated that the protein kinase Akt acts as a co-adaptor in this complex to promote their recycling. This is a fundamentally different function in which Akt kinase acts as an effector rather than a regulator in a cellular event.
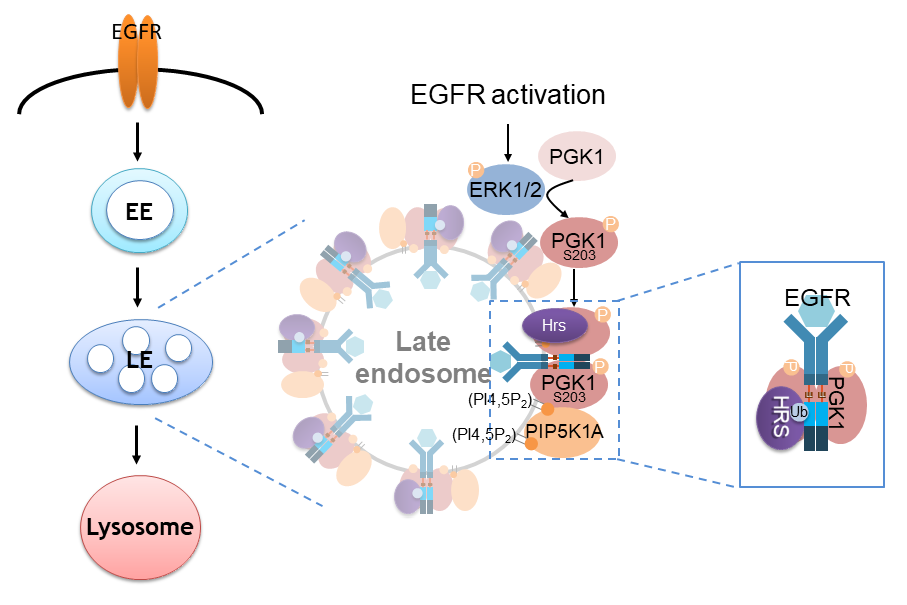
Phosphorylation of PGK1 on S203 promotes its recruitment to endosomal membranes where it acts as a cargo adaptor to recognize the dileucine sorting motif on EGFR to accelerate its transport to the lysosome. This phosphorylation promotes PGK1 recruitment to endosomal membranes in two complementary ways, a protein-based mechanism that involves binding to hepatocyte growth factor receptor substrate (Hrs) and a lipid-based mechanism that involves binding to PI(4,5)P2. These findings not only reveal a previously unknown role of a metabolic enzyme, but also advance the mechanistic understanding of how endocytic EGFR is transported to the lysosome.