本所呂桐睿所長及何孟樵老師與跨國合作的實驗團隊,其研究成果熱騰騰地發表在頂尖期刊《細胞 CELL》!
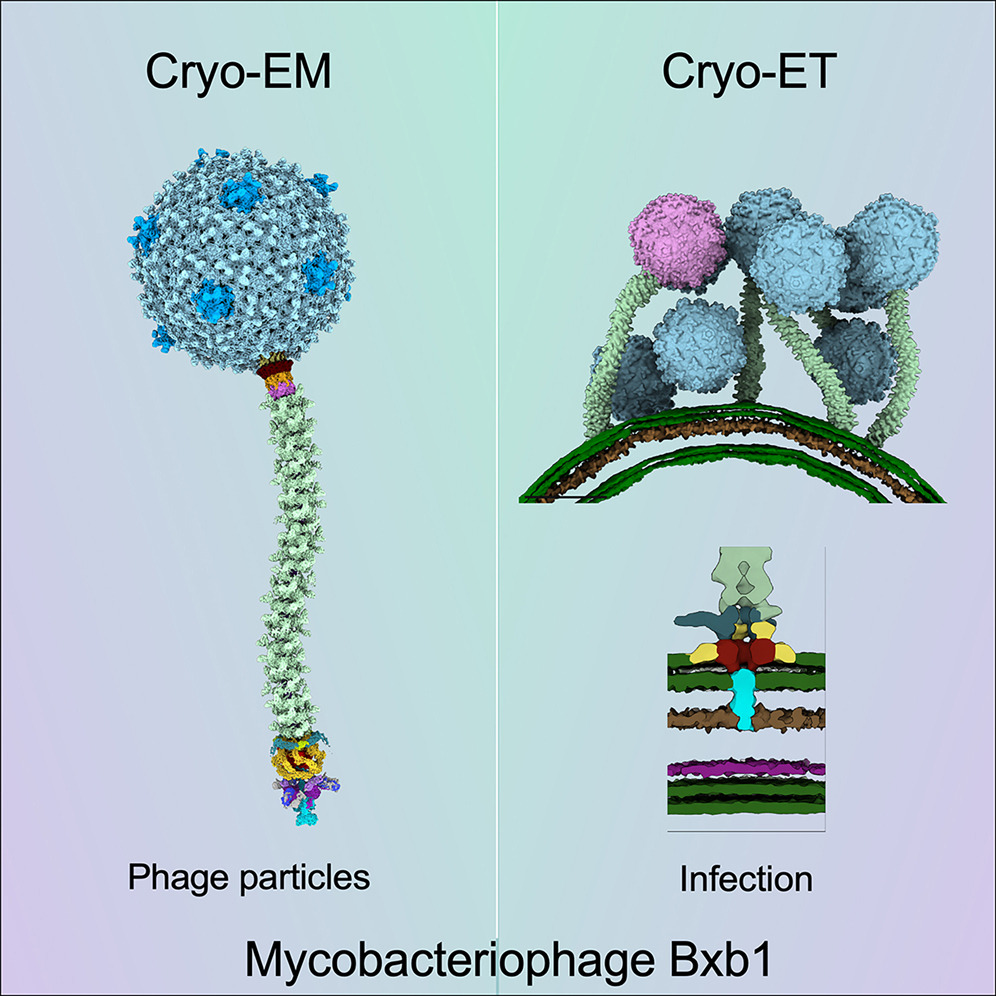
Mycobacteriophage Bxb1 is a well-characterized virus of Mycobacterium smegmatis with double-stranded DNA and a long, flexible tail. Mycobacteriophages show considerable potential as therapies for Mycobacterium infections, but little is known about the structural details of these phages or how they bind to and traverse the complex Mycobacterium cell wall. Here, we report the complete structure and atomic model of phage Bxb1, including the arrangement of immunodominant domains of both the capsid and tail tube subunits, as well as the assembly of the protein subunits in the tail-tip complex. The structure contains protein assemblies with 3-, 5-, 6-, and 12-fold symmetries, which interact to satisfy several symmetry mismatches. Cryoelectron tomography of phage particles bound to M. smegmatis reveals the structural transitions that occur for free phage particles to bind to the cell surface and navigate through the cell wall to enable DNA transfer into the cytoplasm.